Gemstone Glossary
Absorption spectrum
 |
Adularescence
 |
amorph
A mineral is amorphous if it shows the same physical behaviour in all directions. There is internal order of the atoms and molecules. Amorphous material can alter its internal structure and become crystalline, but this is uncommon (opal into chalcedony).
Asterism
 |
Photo (star ruby) by kind permission of © www.ahernbrucker.com.
Birefringence ( Δn)
Birefringence (double refraction) is the difference between the highest and lowest refractive index of a mineral. The higher the difference the more a ray of light, which enters a crystal, splits into different beams: fast and slow resp. ordinary (no) and extraordinary (ne).
Formula: Δn = ne - no
Websites use different symbols for their data:
www.handbookofmineralogy.org: Δn = — , no = ω, ne = ε
www.webmineral.com: Δn = bire, no = w, ne = e
www.mindat.org: Δn = δn , no = nω, ne = nε
These two beams exit the crystal in different angles, affected by the speed. As a result one can see an object below the crystal (or through the crystal) as double.
Especially Calcite and Moissanite show a strong birefringence. Double ("bi") refraction can be measured with a refractometer.
Besides a few minerals some plastics are also birefringent: cellophane, polystyrene and polycarbonate.
See: "Refraction"
⇐ Intro Page
Botryoidal
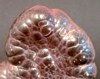 |
Brilliance
Brilliance is caused by the reflection and refraction of the light. It is an important factor for valuing a stone: The more brilliant the more valuable. Brilliance depends on the stone's clarity, cut, and the light source. The smaller the crystals the less brilliance appears: Minerals with a micro crystalline structure are not brilliant. Inclusions lower the brilliance. Special cuts can focus the light towards the eye of the viewer.
Highly brilliant stones are: Moissanite (larger crystals are synthesized), diamond, cubic zirconia (synthetic), rutile and demantoid.
Brilliant-Cut
 |
The modern "round brilliant-cut" has 32 facets plus 1 table facet on the crown (above the girdle), and 24 - 25 facets on the pavilion (lower part, below the girdle). Today a lot of different cuts are used.
See: "Cut"
Cabochon
 |
For some gemstones a cabochon cut is necessary to show a special effect like adularescence, a cat's eye or a star (see e.g. "star sapphire") which would not be visible in a faceted stone.
Cameo
 |
Most modern cameos are carved into agates because of the layers' strong color contrasts. "Two layer stones" can be white on black or blue or brown. Rarer "three layer stones" also exist. Translucent layers allow the creation of shading effects.
⇐ Intro Page
Carat (ct, plural: cts)
Since 1875 the term "carat" is the international standard for the weight of gemstones. 1 ct is equivalent to 0.2 grams. "Carat" originates from the Greek word "keration", for the fruit of the Carob tree (Ceratonia siliqua). Because of the identic weight and size of the carob seeds (0.2 g) already Greeks, Phoenicians and Romans used them as weights.
(ct / diameter) relation for diamonds (density approximately 3.5):
0.10 ct = 3.0 mm
0.25 ct = 4.1 mm
0.50 ct = 5.2 mm
1.00 ct = 6.5 mm
2.00 ct = 8.2 mm
The term carat is also used for the standard of gold and gold alloys but is not a weight measure (ct, kt, K). Pure gold has 24K, which means 24/24 gold shares. One can also express this as 999/1000, which means that the 999‰ stands for the technical and thus financial problem to remove all "soilings" (platinum metals, silver, etc) from the gold.
Gold hallmarks (numerical marking, North American and British marking):
Gold 999 = 24K = 24/24 = 999.9/1000 = 99.99% gold shares
Gold 990 = 24K = 24/24 = 990.0/1000 = 99.00% gold shares (UK)
Gold 916 or 917 = 22K = 22/24 = 916.6/1000 = 91.66% gold shares (UK, Turkey, India)
Gold 875 = 21K = 21/24 = 875/1000 = 87.5% gold shares (Europe)
Gold 750 = 18K = 18/24 = 750/1000 = 75% gold shares
Gold 585 = 14K = 14/24 = 585/1000 = 58.5% gold shares
Gold 416 or 417 = 10K = 10/24 = 417/1000 = 41.7% gold shares (North America)
Gold 375 = 9K = 9/24 = 375/1000 = 37.50% gold shares (North America, UK)
Gold 333 = 8K = 8/24 = 333/1000 = 33.33% gold shares (Germany)
Cat's Eye Effect
 |
The cat's eye effect is also known as chatoyancy. This expression comes from the French "æil de chat" which means "cat's eye". It comes from the structure of a mineral which fibrous inclusions are parallel to the base of the stone. The light is reflected by the vertical direction of the fibres.
Mainly apatite, aquamarine, chrysoberyl, moonstone, quartz, scapolite, and tourmaline show this effect.
Photo by kind permission of © www.multicolour.com.
Chatoyancy
See: "Cat's Eye Effect"
Cleavage
Crystals with "cleavage" cleave (split) in certain directions due to their crystal structure. If crystals have no cleavage, they simply break. There are typically microscopic cracks or cleaves that can be seen under magnification. The gemstone cutter always has to consider the cleavage mechanism of a mineral. Topaz and apatite have strong cleavage planes, while quartz has none. Cleavage increases the difficulty of cutting.
Cleavage is categorized into "perfect", "good", "poor", and "none".
"Perfect" does not leave a rough surface on the broken crystal, "good" mostly leaves a plain surface, "poor" leaves a rough surface, "none" results in an extremely broken, rough surface.
Cluster
"Cluster" means an acumulation of crystals. The typical crystal habit must not be visible.
See: Poldervaartite
Color
Gem colors can be caused by the mineral's intrinsic color, impurities in the part per million level, foreign inclusions, or crystal structure. One can distinguish between intrinsic coloring and other sources using various tests. The "streak check" can determine if the color is intrinsic (malachite remains green while sapphire is white). The "streak check" is done by scratching the mineral on a rough porcelain plate.
⇐ Intro Page
Conchoidal Fracture
If a material breaks in a way that the fracture does not follow any natural possibilities of separation. The surface of such cracks looks curved, like shells. Conchoidal fracture is typical of fine-grained and amorphous materials like flintstone etc.
See: "Cleavage"
Crystal
A crystal is a chemically and physically homogeneous solid which is terminated by plain and smooth sides, and which has a symmetric internal structure. There are seven crystal systems: triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. The crystal's planes are often not in a typical proportion but the angles between the analogue planes are always the same (legitimacy of constant angles).
Cut
Gemstones show different cuts. The perfect cut depends on the ability of the cutter. If you buy a gemstone by just having seen a photo, keep a close eye on the stone's parallel edges. For exact valuation use the help of an experienced jeweler or a noted gem laboratory.
The modern polyhedron form shows as many facets as possible, which refract and reflect the light as varied as possible. Until the 16th century only the cabochon cut was in use. This cut is used until today for less valuable gemstones with too many inclusions or a lower transparency. An exception is the asterism of some minerals, e.g. star sapphires. In this case the cabochon cut is necessary to show the star, and the transparency usually plays a less important role (except from nearly transparent stones which are usually high valued).
Density ("specific gravity")
To determine the density of a stone one measures its weight with a scales, and its volume with a graduated cylinder. Then one divides the grams through the cm3.
Deposit
 |
Gemstone deposits are irregularly distributed on earth. Some rich focal points are the Rocky Mountains (USA), Minas Gerais (Brazil), Ural mountains (Russia), Myanmar, south and east Africa, and possibly Antarctica.
Dispersion
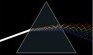 |
Photo: Gif animation > 1 MB! Author: Lucas V. Barbosa
See: "Refraction"
End-members / mineral series
End-members are minerals which are at the end of a series of minerals. Within a mineral series the pure minerals (which have a specific formula) can be mixed among another, resulting in various different types. Pure end-members are practically not existing in nature but can be synthesized. Within the borders of the end-members the chemical composition can vary without changing the structure of the formula resp. the crystal structure.
There must not be only two end-members. For example, there are three end-members in the pyralspite garnets series: Almandine Fe3Al2(SiO4)3, pyrope Mg3Al2(SiO4)3 and spessartine Mn3Al2(SiO4)3. One can see that a specific place within the molecule can be occupied by varying quantities of the metal ions iron (Fe), magnesium (Mg) and manganese (Mn).
Another well-known example of a mineral series is feldspar with the end-members albite NaAlSi3O8, anorthite CaAl2Si2O8 and K-feldspar KAlSi3O8.
Fluorescence
Fluorescence is a sort of photoluminescence with a very short time of light emission. It occurs when a molecule or atom relaxes to its ground state after having been electrically excited. That means that e.g. a mineral emits light in a different color than before after having been exposed to e.g. UV light.
Minerals can show a distinctive fluorescence or fluoresce differently after exposure to short-wave ultraviolet light (SW UV), medium-wave ultraviolet light (MW UV), long-wave ultra violet light (LW UV) or X-rays.
For examples see Tugtupite.
Fracture
See: "Cleavage"
Gemology
Scientifically dealing with gemstones.
⇐ Intro Page
Gemstone
A mineral (also organic material as pearls, corals, and amber), distinguished by the combination of as many as possible of these properties: Great lustre, transparency, beautiful color, hardness, strong dispersion, resistance, rarity, and sufficient size of the crystals for the production of jewelry.
The value of gems also depends on the mode and country-specific preferences. Gemstones appear in different mineral groups.
Hardness
Durability of a mineral against penetrating of a material into it. Amongst the international gemology society the Mohs (German gemologist, 1773 - 1839) scale is widely used which compares 10 minerals, depending on their ability which scratches which. The Mohs scale reaches from talcum (hardness 1) to diamond (hardness 10). Because the hardness of minerals doesn't increase regularly, other scientists have invented different scales (see my page Mohs Scale) which considers the depth of penetration.
Inclusion
An inclusion is a foreign element within a crystal, mostly gas, fluid or another mineral. Synthetic crystals usually don't contain any inclusions. Inclusions usually reduce the value of a gemstone, depending on the visibility by the naked eye or a loupe (10 times). Sometimes inclusions are an indication on the locality, e.g. the "horse-tail" inclusions in Russian demantoids which even increase their value in comparison with demantoids from other locations. Inclusions can also cause sought light effects like asterism.
K
See: "Carat"
Labradorescence
 |
Photo by kind permission of © http://skywalker.cochise.edu/wellerr/, Roger Weller.
Luminescence
Appearance of a mineral in ultraviolet light. If one irradiates certain minerals with ultraviolet light in the darkness, they partionally flash in different colors. If this strange gleaming is continuing for a while, after switching-off the lamp, it is called phosphorescence. Depending on the deposit, the same mineral shows luminescence or not (e.g. hauyne), depending on the content of foreign metals or trace elements. Some minerals glow when heated ("thermoluminescence", e.g. fluorite).
For gemological inspections only two shortwave ranges play a role: shortwave UV light (100 nm - 280 nm), and longwave UV light (315 nm - 400 nm). Fluorescent inspection helps detecting forgeries. For inspections of pearls X-rays are used which show typical luminescence of culture pearls, natural pearls, sea water pearls and fresh water pearls.
Luster
Luster depends on the smoothness of a crystal's or a cutted gemstone's surface. The more light is reflected the more lustrous a stone appears. Luster types:
Metallic: Highly reflective (e.g. pyrite)
Sub-metallic: Slightly less reflective than metallic (e.g. magnetite)
Adamantine: Top brilliant (e.g. diamond)
Vitreous: Shiny like broken glass (e.g. quartz)
Pearly Like the surface of pearls, caused by thin layers beneath the surface (e.g. muscovite)
Resinous Like resin (e.g. amber)
Silky A mineral reflects a soft light because of its fibrous aggregates (e.g. gypsum)
Dull No reflections because of microscopic crystals (e.g. chrysocolla)
Mineral
Anorganic material or chemical element which can be defined by a special chemical formula and crystal structure. Minerals often contain inclusions of gases, liquids or other minerals which show the natural origin (crystal growth).
⇐ Intro Page
Mineral Class
Minerals are usually classified according to their chemical composition and crystal structure. Today the classicication after Hugo Strunz is widely used. Strunz invented 9 mineral classes:
1. elements
2. sulfides
3. halogenides
4. oxides and hydroxides
5. nitrates, carbonates, and borates
6. sulfates, chromates, molybdates, and wolframates
7. phosphates, arsenates, and vanadates
8. silicates
9. organic compounds
Mineral series
See: "End-member / mineral series"
Opalescence
A type of dichroism in translucent, cloudy, highly dispersed systems in which very small particles (partially as small as the wavelength of light) are suspended. The material appears yellowish-red in transmitted light because the longwaved, red parts of the sunlight are less scattered at the particles than blue light (shorter wavelength). For example opal or milky water look yellowish-red in backlight but blueish when seen from aside, in front of a dark background.
Opalizing
Multicolored and limited colors on the surface of opals which change depending on the angle of view resp. "wander" across the surface. Ideally all colors of the spectrum appear. Reason for this effect are very small balls of the mineral cristobalite which reflect the light in interference colors ("rainbow colors").
Phosphorescence
 |
Photo by kind permission of © www.minershop.com.
Pleochroism
 |
Pleochroism can appear from weak to strong. The gem cutter has to consider the pleochroism so that the finished stone shows the desired brightness and color.
Photo by kind permission of © www.irocks.com.
Rarity
The relation between supply and demand makes a gemstone's price. Most respected and wished are the traditional gemstones: diamond, ruby, sapphire and emerald. The cleaner and larger a stone the more money one has to pay for it. Besides that the color of a stone can increase its value. E.g. "pidgeon blood" rubies are much more valuable than rubies with other red colors.
Only parts of raw but gemmy crystals are usually eye-clean. Therefore the cutter must try to save as much clean material as possible to create the maximum size of the gemstone. That's the reason why symmetrical gemstones (round cut, emerald cut, trilliant cut etc.) are more valuable than those (equal weight) having an asymmetrical cut, and larger stones more valuable than smaller ones.
A lot of facetable minerals are even much rarer than the classic gemstones and sought after by collectors. Because of their limited occurrence their prices can reach some thousand $US/ct. Even if a gemmy mineral occurs at several locations (like Hauyne) and therefore isn't very rare itself, large crystals resp. faceted stones of 1 ct or more are very rare and expensive.
Even heavily included but transparent crystals are very much sought after by collectors because of their rarity. An example is Chambersite whose usually small crystals only appear in brine returns from gas storage wells in Texas / USA.
Refraction
 |
Formula: n = c0 : c
n = refractive index, c0 = speed of light in the vacuum, c = speed of light in the examined substance (e.g. quartz).
Some refraction indices: Amethyst: 1.543 - 1.554; uvarovite: 1.86 - 1.87; diamond: 2.417 - 2.419.
There are different refractive indices within a crystal, depending on the crystal system. Amorphous materials have just one refractive index. The measurement of the refractive index (with a refractometer) is an important aid regarding the identification of a mineral resp. a gemstone.
See: Refractive Indices
⇐ Intro Page
Synthetics and imitations (history)
2000 b.c.: Egypts produced imitations of gemstones by using glass and enamel.
1758: Joseph Strasser (Vienna) created a sort of glass which could be cutted. It looked similar to diamonds ("Strass-Stones").
Around 1830: First synthetic gemstones.
Around 1890: A.V. Verneuil created a method for production of synthetic rubies ("Melting drops method").
1910: A.V. Verneuil started with the production of synthetic sapphires, later with corundum (ruby and sapphire) in different colors.
1926: Production of synthetic spinels with the Verneuil method.
1947: First synthetic star rubies and star sapphires in the USA.
Around 1947: Synthetic emeralds.
1948: Synthetic rutiles.
1955: First synthesis of diamonds in the USA and Sweden.
1970: Diamond synthesis in gem quality.
1977: Creation of an artificial gemstone (yttriumcirconoxide), called "Circonia".
Artificial gemstones have to be declared as artificial if traded.
Tenebrescence
Tenebrescence (photochromism) is the reversible reaction of a substance related to light (daylight, UV light, laser light) exposure. Like the well-known photochromic eyeglass lenses which change their color in dependence on the intensity of the sunlight, also minerals can be tenebrescent. See Tugtupite or Hackmanite.
Transparency
Minerals let the light pass through differently. The value of a gemstone usually increases with its transparency. One distinguishes between transparent (water clear), translucent (light can pass through a relatively thin slice), and opaque (light cannot pass not even a relative thin slice).
UV Light
Light is electromagnetic radiation of all wavelengths (in the broader field of physics). Visible light has a wavelength between ~380 and ~750 nm (nanometers: unit of length, 10-9 m, a thousand-millionth of a meter).
UV light, which is not visible for human beings, has a wavelength beyond our visible spectrum which reaches from red (longest wavelength) via orange, yellow, green and blue to violet (shortest wavelength). Some animals (e.g. insects) are able to see UV light. X-rays have an even shorter wavelength than UV light.
UV light is an important part of the radiation received from the sun, causing e.g. sunburn. Fluorescent minerals like tugtupite react on UV light which can also be produced by special lamps. One distinguishes between
short-wave UV light (SW UV or UV "C") with 100 nm - 280 nm
medium-wave UV light (MW UV or UV "B") 280 nm - 315 nm and
long-wave UV light (LW UV or UV "A") 315 nm - 400 nm.
© RealGems.org 2007 - 2011
⇐ Intro Page